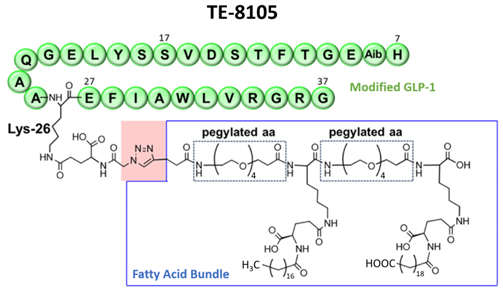
Innovative Long-acting GLP-1 Design Significant Extends Half-life
GLP-1 is an incretin hormone that stimulates insulin secretion, suppresses glucagon, delays gastric emptying, and reduces appetite through central nervous system pathways. However, its therapeutic use is limited by a natural plasma half-life of only approximately two minutes. TE-8105 is engineered using Immunwork’s proprietary “Fatty Acid Bundle Platform”, combining a modified GLP-1 peptide with a fatty acid bundle to markedly prolong its half-life. This long-acting profile enables once-every-one-to-two-weeks dosing, offering improved convenience and a new therapeutic option for chronic metabolic diseases.
Positioned for a Rapidly Growing Obesity Drug Market
With GLP-1–based therapies moving beyond diabetes into obesity treatment, the global weight-loss drug market continues to expand rapidly. The market size is projected to increase from USD 13.84 billion in 2024 to USD 48.84 billion by 2030, with a CAGR of 18.54% (Source: Grand View Research). Leveraging its long-acting profile and innovative technology, TE-8105 is well positioned as a promising candidate for obesity, weight management, type 2 diabetes, and fatty liver disease.
Phase 1/2a Results Confirm Safety and Tolerability
The Phase 1/2a clinical program for TE-8105 consisted of two parts:
Part A – Single-ascending-dose study: Four dose groups (0.5 mg, 0.75 mg, 1.5 mg, and 3 mg), each enrolling six overweight or obese adults without diabetes.
Part B – Multiple-ascending-dose study:
B1 (n=6): Five doses of 1 mg TE-8105 every two weeks, followed by an 11-week observation period.
B2 (n=8): Nine escalating doses—1.5 mg (2 doses), 2 mg (2 doses), 2.5 mg (2 doses), and 3 mg (3 doses) every two weeks, followed by a 6-week observation period.
TE-8105 demonstrated excellent tolerability, with fewer than 20% of participants experienced mild-to-moderate gastrointestinal (GI) symptoms and no severe adverse events reported. The dose-escalation design effectively minimized GI side effects.
Metabolic Improvement Highlight Therapeutic Potential
TE-8105 treatment also showed improvement trends across multiple metabolic indicators, including body weight, BMI, HbA1c, and waist-to-hip ratio, underscoring its therapeutic potential for obesity management. Notably, five participants in the B2 cohort exhibited a robust response to TE-8105, each achieving more than a 5% reduction in body weight during the study period. Among the three prediabetic participants in the B2 cohort (baseline HbA1c 5.7–6.4%), all showed reductions in HbA1c after treatment, with two returning to normal glycemic range (<5.7%), highlighting TE-8105’s potential to improve obesity-related metabolic dysfunction.
Phase 2b Development to Advance Clinical Strategy
Immunwork plans to initiate the Phase 2b clinical trial in the second half of 2026, aiming to optimize the dosing regimen and further assessing the therapeutic benefits of TE-8105 in obesity and associated metabolic diseases. Immunwork will continue exploring additional indications to maximize TE-8105’s clinical value and deliver safer, more accessible treatment options for patients living with overweight or obesity.
1. https://news.gbimonthly.com/tw/article/show.php?num=82213&range=news
2. https://www.ctee.com.tw/news/20251208701280-430503
3. https://money.udn.com/money/story/5612/9190829?from=edn_related_storybottom

