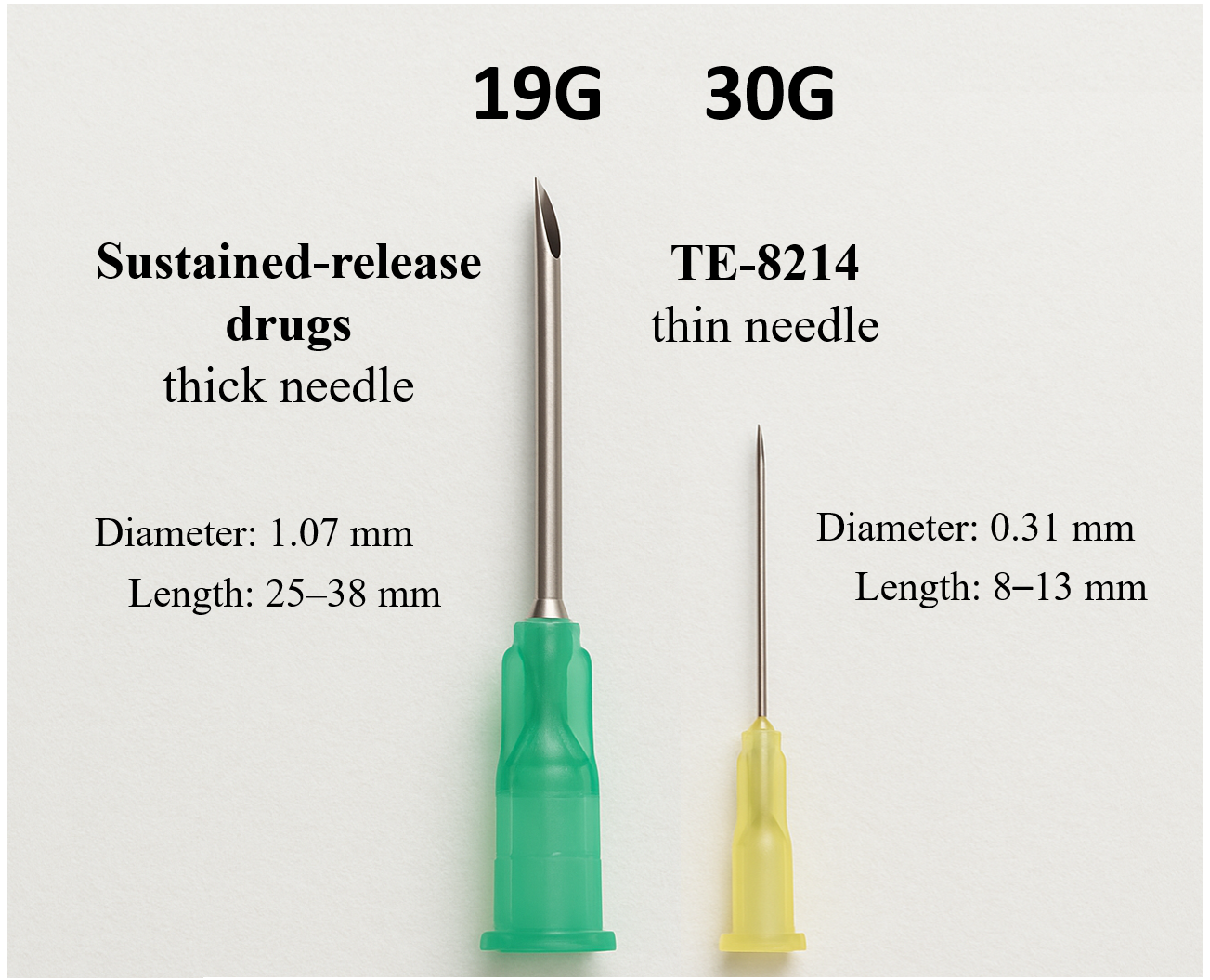
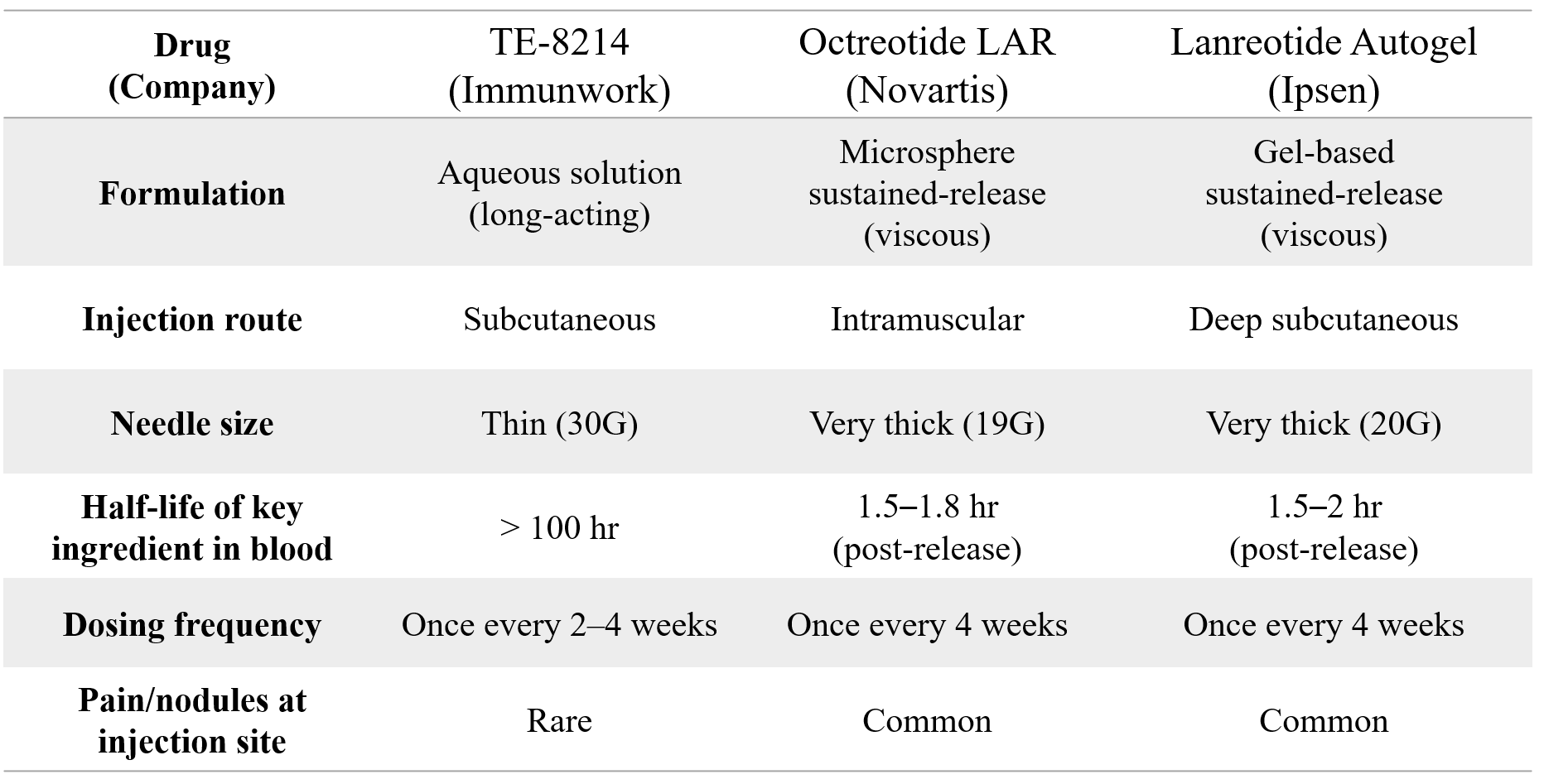
• Pioneering Patient Experience: TE-8214, a highly water-soluble aqueous solution, enables pain-free subcutaneous injection with a thin 30G needle, eliminating injection site reactions seen with current therapies.
• Strong Pharmacodynamic Activity: Robust, dose-dependent reduction of disease biomarker IGF-1, with 83% of high-dose participants achieving a >20% reduction, confirming therapeutic potential.
• Significant Market Potential: Positioned for the US$10 billion long-acting octreotide market by addressing key unmet needs of painful injections and side effects associated with current viscous, thick-needle formulations.
• Phase II Initiation and Strategic Partnering: Phase II trial planned for Q3 2025; Immunwork is actively seeking strategic collaborations to accelerate global development and commercialization.
Immunwork, Inc., a clinical-stage biotechnology company developing transformative therapies, today announced positive top-line results from its Phase I clinical trial (in Australia) of TE-8214, a novel long-acting octreotide analog for the treatment of acromegaly and neuroendocrine tumors (NETs). The study met its primary objectives: TE-8214 demonstrated good safety, tolerability, and pharmacological activity, with pain-free subcutaneous administration and no injection site reactions.
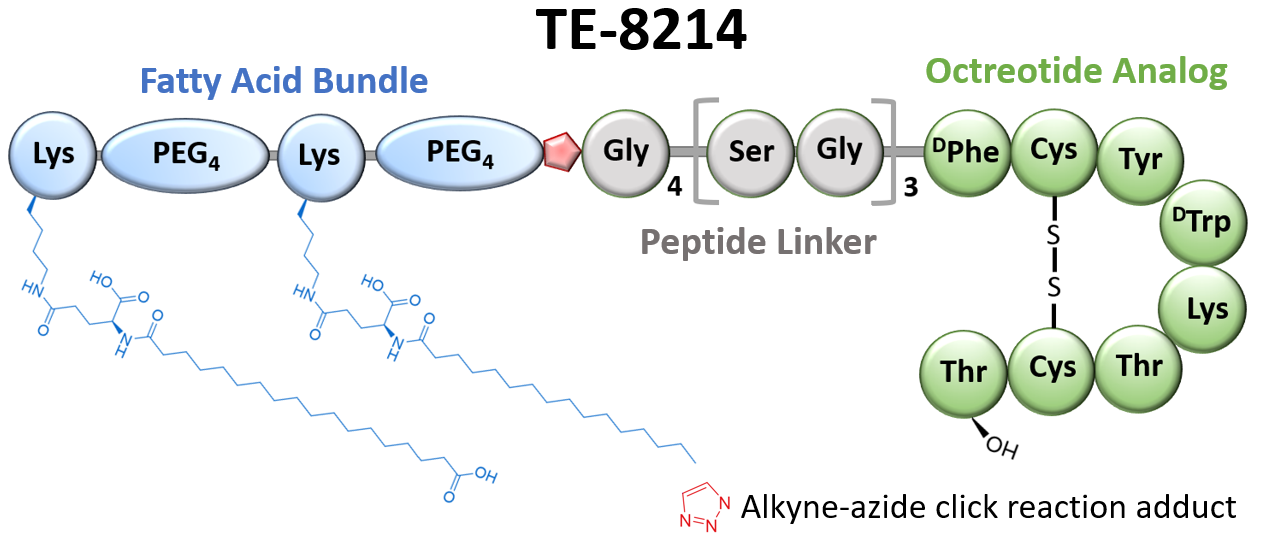
A Next-Generation Therapy for Unmet Patient Needs
TE-8214 was developed using Immunwork’s proprietary fatty acid modification platform, which extends the drug's half-life and increases water solubility. TE-8214’s water-soluble, low-viscosity formulation allows for subcutaneous administration with a fine 30G needle, offering a dramatically improved patient experience compared to the current standard-of-care, which requires thick, viscous formulations administered with large 19G or 20G needles that often cause significant pain and injection site reactions. This innovation addresses a critical need for safer, more patient-friendly long-acting therapies in a global octreotide market projected to reach US$10 billion by 2030.
Phase I Clinical Highlights TE-8214’s Differentiated Profile
The randomized, double-blind, placebo-controlled, single-ascending dose study enrolled 32 healthy subjects (24 receiving TE-8214 and 6 receiving saline) across four dose cohorts (0.6, 1.2, 2, and 4 mg).
Exceptional Safety and Tolerability: TE-8214 was well tolerated with no serious adverse events reported. Notably, no TE-8214 recipients experienced injection site pain or nodules —results identical to placebo with saline — demonstrating a clear advantage over existing therapies. Gastrointestinal side effects, often seen with octreotide, were minimal: only 3 of 24 TE-8214 recipients reported very mild gastrointestinal discomfort.
Potent and Sustained Pharmacodynamic Activity: Octreotide drugs mainly exert their therapeutic effects by suppressing insulin-like growth factor 1 concentration in the body. In the two highest dose cohorts (2 mg and 4 mg), 10 of 12 participants (83%) achieved a >20% reduction in insulin-like growth factor 1, confirming robust biological activity and validating its therapeutic potential for Phase II studies.
“These Phase I clinical trial results confirm TE-8214’s differentiated profile: TE-8214 is highly water-soluble, can be administered subcutaneously using a fine needle, significantly reducing injection pain and side effects for patients,” said Dr. Tse-Wen Chang, founder and CEO of Immunwork and a pioneer of anti-CD3 (OKT3) and the inventor of anti-IgE (Xolair) for asthma and allergy. “By solving the fundamental formulation and administration challenges of current treatments, we have created a product with the potential to become the new standard of care. TE-8214’s profile—combining ease of administration, superior tolerability, and strong efficacy—positions it to capture a significant share of the market and, most importantly, vastly improve the quality of life for patients.”
Strategic Outlook: Advancing to Phase II and Seeking Partnerships
Immunwork is preparing for a Phase II trial of TE-8214 in Taiwan, planned to start in Q3 2025, to further evaluate the efficacy and safety of TE-8214 in patients. The company is actively pursuing strategic partnerships with pharmaceutical leaders to accelerate late-stage clinical development, navigate global regulatory pathways, and maximize the commercial potential of TE-8214.
TE-8214: A Differentiated Profile vs. Marketed Long-acting Octreotide Analogs
Related News Website Link:
1. https://www.ctwant.com/article/427203/?utm_source=share&utm_medium=mobile
2. https://news.pchome.com.tw/living/crwant/20250701/index-75133520059225316009.html
3. https://news.gbimonthly.com/tw/article/show.php?num=78270
4. https://www.ctee.com.tw/news/20250701700820-430504
5. https://today.line.me/hk/v3/article/gzY821a
6. https://www.styletc.com/article/428059

